Deuterium Depleted Water
Deuterium Depleted Water
The Water with the Lowest Deuterium Content on Earth
Deplete deuterium to optimize cellular energy.
Drink Light Water daily to reduce deuterium interference.


Deuterium (heavy hydrogen) is heavy.
What is Deuterium?
All water on Earth, including seawater, river water, and drinking water, contains about 145-155 ppm of deuterium.
Additionally, plants, animals, and the human body contain an average of 155 ppm (parts per million) of deuterium.
It has been physiologically revealed that when aging or immunity becomes unbalanced, deuterium in the human body acts as a growth factor for abnormal cells, such as inflammation and tumor cells.
Hydrogen is the most common element on Earth and exists as a molecule in the food we eat and the water we drink.
The hydrogen in the food we artificially inhale or ingest acts on our blood, organs, and DNA, contributing to antioxidant effects. However, deuterium is a rare type of hydrogen.
Deuterium (heavy hydrogen) uses the first character “중 (重),” meaning “heavy,” because it has one more neutron compared to regular hydrogen, making it a heavier form of hydrogen.
Water that contains a high level of deuterium has no significant difference in taste or appearance from regular water, but it has an important chemical difference.
Regular water boils at 100°C, while heavy water boils at 101.4°C. Regular water freezes at 0°C, while heavy water freezes at 3.8°C. As seen in the video, ice made entirely from deuterium water sinks immediately in water.
Isotopes of Hydrogen: Deuterium and Tritium?
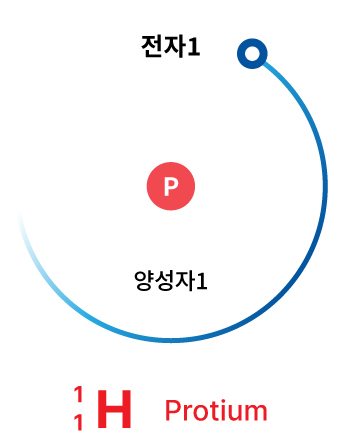
Hydrogen
Exists on Earth along with water. Ho₂

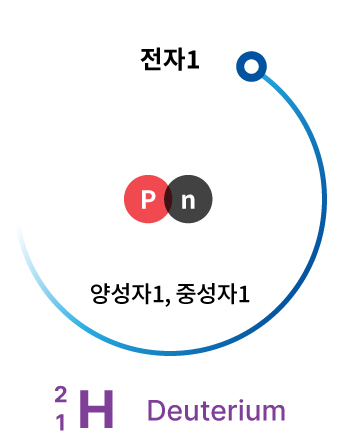
Deuterium
Hydrogen contains only one proton in its nucleus.
In contrast, deuterium has one proton and one neutron in its nucleus. Deuterium is commonly used in nuclear reactors to slow down neutrons. It is not a radioactive substance.
In a human body weighing 75 kg, there is about 50 kg of water, and approximately 10 g of deuterium (about 1/6000 of the water or 150 ppm on average) exists in the body.
Physiological clinical trials with an increased proportion of deuterium have shown that if 25% of the water in a mouse or dog’s body is deuterium, they become infertile, and if it reaches around 50%, they survive for only about a week. Moreover, it has been confirmed that fish and tadpoles die immediately in water containing 90% deuterium.
Deuterium is also currently used in the production of OLED blue light sources. Additionally, it is utilized in nuclear magnetic resonance spectrometers (NMR).
Source: Naver Knowledge Encyclopedia – Physics
https://terms.naver.com/entry.naver?docId=3537278&cid=60217&categoryId=60217
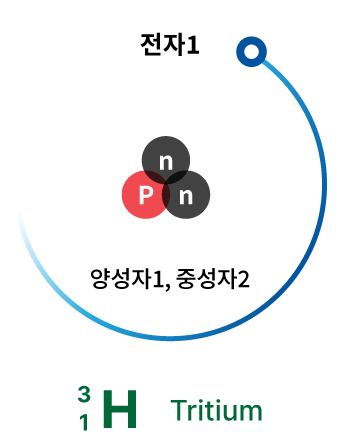
Tritium
The radioactive substance being released into the ocean from the Fukushima nuclear power plant in Japan is tritium.

2L, Deuterium Content 10 ppm

1L, Deuterium Content 78 ppm
