What Is Water, Really?
While we think we know what water is, few people truly grasp its complexities. Water is everywhere, and we often take it for granted. And just as we rarely question the air we breathe, we do not question water; therefore, our general knowledge of water remains limited despite its fundamental role in life.
What Are the Components of Water?
Water is a compound made up of oxygen and hydrogen, consisting of two hydrogen atoms and one oxygen atom. The molecular formula for water is H₂O, and the basic structure of any water is the same. Water tends to maintain a constant concentration over time, temperature and circulation by mixing with various minerals and organic matter around it. Water also has dissolved oxygen from the air due to atmospheric pressure, and the oxygen concentration varies significantly depending on temperature and pressure. Because of this, water is sometimes regarded as a mysterious and almost magical substance.
Deuterium-depleted water is not water that had its minerals or organic matter altered. Deuterium-depleted water refers to water that naturally maintains a lower concentration of deuterium (²H), a hydrogen isotope that exists in water at an average concentration of 155 ppm. In other words, deuterium concentration of water that people drink and cook with everyday is, on average, 155 ppm.
Many scholars have studied why people in the world’s longevity villages live longer and age slower. They found no difference in the water from these villages than regular water. This raised the question, “If the water quality is the same, why don’t we live as long?” Some attempted to explain it through food or dietary habits, but no clear evidence was presented.
However, scholars turned their attention to a new factor that had not been included in the analysis. It was found that water in longevity villages had a significantly lower deuterium concentration, averaging 130 ppm, compared to regular water. Research on the role of deuterium in the human body has shown that deuterium plays a significant role in the process of cell division and is crucial for promoting cell differentiation. This may be one of the reasons why people in longevity villages age slowly and live longer.
Subsequent studies on longevity villages analyzed the deuterium concentration in the water from Lake Titicaca in Bolivia and the crops around the Hunza Valley. It was found that the plants also had low deuterium concentrations. The lower the deuterium concentration, the slower the rate of cell division, leading to the conclusion that people in longevity villages look younger, are healthier, and are less susceptible to diseases compared to their age.
Deuterium-depleted water plays a crucial role in anti-aging. Clinical results have consistently shown that long-term consumption of water with low deuterium concentrations can affect the division rate of cancer cells, causing them to either stop or naturally undergo apoptosis (self-destruction).
Meanwhile, deuterium-depleted water is also known as “heavy water,” and when the concentration of heavy water is reduced, the water becomes lighter, called “light water”. The average deuterium concentration in natural water on Earth is 155 ppm, “light water” has around 100-150 ppm, “super light water” has 50-100 ppm, and “ultra light water” has below 50 ppm.
Naturally, the distribution of deuterium concentration varies with altitude. The higher the altitude, the lower the deuterium concentration, and the lower the altitude, the higher the concentration. This characteristic is evidenced by the fact that longevity villages are typically located in high-altitude areas above 3,000 meters.
While it is possible to produce low-deuterium water using this natural distribution, it is a very challenging task. In the past, Japan invested more than 2 billion yen to build a deuterium-depleted water production plant, but the daily production was so low that the factory had to close due to economic infeasibility. This demonstrates that producing deuterium-depleted water is a very complex and difficult process.
As a result, deuterium-depleted water is sold at a much higher price than regular water.

The Secret of Longevity
In the summer of 1988, an elderly man passed through immigration control at London’s airport. The immigration officer was astonished upon checking the man’s passport, as his age was recorded as 160 years old.
The man’s nationality was a village called Hunza, located beneath the Karakoram Mountains in Pakistan. This village is known for stories of women in their 40s looking like teenagers, women in their 60s frequently giving birth, and even a woman in her 90s has given birth. The Hunza longevity village in Pakistan, with an average life expectancy of 120 years, became very famous.
The village of Hunza, situated at high altitudes in the Himalayas, was completely isolated from the outside world, and until the early 1990s, no one in the village suffered from cancer, heart disease, or degenerative illnesses. It is also well-known that people living in mountainous regions around the world tend to live longer lives.
Researchers studying aging have pointed to the low deuterium content in the water they drink as one of the reasons for the longevity of people in high-altitude regions with perpetual snow. While factors like food, lifestyle, and environment are important, the significance of the water they drink and use in their cooking cannot be neglected. Water from melted glaciers contains about 15% less deuterium than regular water, which positively affects tissues and cell membranes, contributing to beneficial effects on metabolism.
In regions located at altitudes of 3,600 to 4,000 meters, crops such as barley, wheat, potatoes, tomatoes, and corn also have low deuterium concentrations. Particularly, mulberry fields exist at altitudes above 3,700 meters, and this area is also known as a longevity village. It is noteworthy that almost no cases of cancer or deaths from cancer have been recorded in these areas.
Additionally, sheep and yaks graze at altitudes above 4,000 meters, so the people who live long lives in these regions consume meat from animals raised on low-deuterium water. In this way, they are surrounded by an environment rich in low-deuterium content, contributing to their longevity.
The History of the Medical Discovery and
Evidence of Deuterium-Depleted Water
13.8 billion years ago, the universe’s first element created from the primordial plasma was hydrogen, composed of one proton and one electron. At the time of the Big Bang, the temperature of the universe was approximately one billion degrees, and electrons and protons intertwined, sometimes annihilating each other and creating photons. Simultaneously, protons and neutrons combined to form deuterium, a pair consisting of a proton and a neutron.
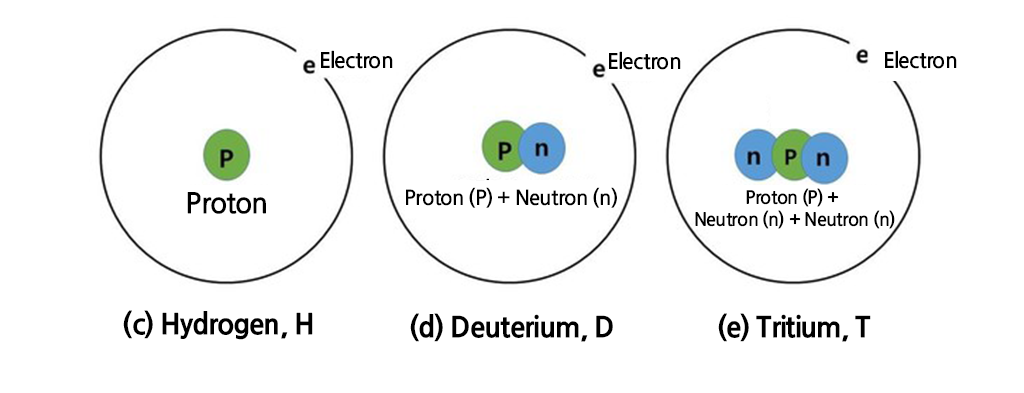
As nearly all deuterium combined to form helium, the primordial matter was divided primarily into 1/4 helium and 3/4 hydrogen. Then, tritium, an extremely rare radioactive isotope (with 2 neutrons), existed at a ratio of about one tritium atom for every 41 million hydrogen atoms. As the universe cooled, deuterium nuclei remained separate without pairing with hydrogen or helium. Most deuterium was used as an energy source for stars, alongside hydrogen.
Later, deuterium atoms (and regular hydrogen) combined with oxygen atoms in a 2:1 ratio to form water, which is now found in both seawater and freshwater on Earth. Approximately six drops, or about 300 mg, of deuterium are found in one liter of Earth’s water.

Harold Urey

Ferdinand Breckwidde
In 1932, Harold C. Urey and his colleagues Ferdinand G. Brickwedde and George R. Murphy at Columbia University in the United States proved the existence of deuterium.
Before their discovery, it was believed that hydrogen consisted of only one proton and one electron. This rare hydrogen isotope, with an added neutron, is twice as heavy as regular hydrogen, making its mass double that of hydrogen.

Deuterium accounts for approximately 0.0149% of all hydrogen in the universe, which made it difficult for physicists to discover it. In 1913, some physicists suspected the possibility of a second hydrogen isotope, but it was Harold C. Urey who later proved its existence.
In 1934, Dr. Urey was awarded the Nobel Prize in Chemistry for this monumental discovery, which contributed to the dawn of the atomic age. Concentrated deuterium, or heavy water, became a critical element for nuclear reactors and the production of atomic bombs.
The 1930s was a period of paradigm shifts in physics, but molecular biology was relatively slow to develop. Meanwhile, in 1929, it was discovered that all living organisms produce ATP in the mitochondria. This occurred 34 years after Carl Benda proved the existence of mitochondria within cells in 1897.
In 1937, the Krebs cycle, which explains the mechanism of ATP production, was discovered. The Krebs cycle, also known as the tricarboxylic acid (TCA) cycle, describes the second stage of cellular respiration, where the metabolic byproducts of glycolysis (the first stage of oxygen respiration) are oxidized, storing part of the energy in ATP and passing the rest to the electron transport chain. However, it would take another 60 years before the effects of deuterium on ATP production were understood.
Shortly after Urey proved the existence of deuterium, his mentor, Gilbert N. Lewis, a professor in the Department of Chemistry at Berkeley, became the first to produce pure heavy water through electrolysis in 1933. He was also the first to observe that when heavy water freezes, it sinks completely in regular water. In addition, he observed that heavy water inhibits microbial reproduction and delays seed growth. From that point, a new era of research focusing on this newly discovered hydrogen isotope, deuterium, began.
Shortly after Lewis’s observation of heavy water, Oscar W. Richards, a researcher at the Osborn Zoological Laboratory at Yale University, found that yeast and sugar act nine times more slowly in heavy water. From 1934 to 1939, HG Barbour and his colleagues in the Department of Pharmacology at Yale University began the first systematic studies on the effects of deuterium on mice.
Between 1933 and 1939, 216 studies on the biological effects of deuterium were published, and they all reached similar conclusions. In experiments where 30% deuterium water was mixed with regular water, it was observed that bacteria, plants, and animals died within a few days.
Although further research was needed, the outbreak of World War II led to a surge in military demand for deuterium, making it increasingly difficult to obtain. As a result, biological research on deuterium was halted, and such studies gradually disappeared by the 1950s.
In 1953, around the same time that Francis HC Crick and James D. Watson published the double-helix structure of DNA, a graduate student at Tomsk University in Siberia (Soviet Union), Gennady D. Berdyshev, made an important discovery while researching gerontology and genetics. Together with his colleague, biophysicist Boris N. Rodimov, he investigated a unique question about the lifespan of the Soviet Union’s population.
While the percentage of centenarians across the entire Soviet Union was less than 10 per million, in certain areas of Siberia, this rate was 324 per million (32 per 100,000, for reference, in 2019, South Korea had about 25 per 100,000). These regions were the high Altai Mountains and Yakutia, where the residents remained healthy and vigorous into old age. Berdyshev discovered that these areas receive clean, melted glacial water from high altitudes, and investigated the possible link between this water and the residents’ longevity. The scientists focused on the possibility that hidden properties of ancient glacial ice might be related to longevity.
In the first experiment, they extracted ice that had been frozen for 300 million years from a depth of 20 meters and melted it. They observed an anti-aging effect of this water on cell division. But the research institute could no longer afford the cost of extracting 300-million-year-old ice, so the researchers turned their attention to the water in the Siberian region. Surprisingly, this water also showed similar effects. This discovery marked the beginning of full-scale research on deuterium-depleted water.
From 1959 to 1960, VM Muhachev at Tomsk University conducted experiments and tried to convince his colleagues that even small amounts of deuterium could distort the chemistry of hydrogen bonds and inhibit submolecular processes. By 1960, Berdyshev had gathered sufficient information to conclude that human longevity in the Yakutia and Altai regions was linked to the consumption of melted glacial water.
Researchers at Tomsk University discovered that, compared to Vienna Standard Mean Ocean Water (VSMOW), which has a deuterium ratio of 155.76 ppm at the equator, the water from ancient melted ice and glaciers in high mountains showed a 15-20% reduction in deuterium. This historic discovery was first published in the Omsk Agricultural Journal in 1961.
Berdyshev, Rodimov, Muhachev, and others found that deuterium-depleted water played an important role in restoring vitality to the human body. Around this time, they learned of a six-level nuclear accident at the Kyshtym nuclear power plant in the southern Ural Mountains, which was the third-largest nuclear accident in history. Berdyshev and his colleagues provided the newly discovered “miracle ice water” to several of the victims, and they observed remarkable recovery results.
In 1966, Rodimov and his department chair in biology and physics, IV Toroptsev, published their research in English, introducing it to researchers and scientists worldwide. Through their groundbreaking discoveries on the physiological role of deuterium in living organisms, they brought Siberia into the global spotlight. They became the first scientists to show how deuterium-depleted water positively affects biological systems.
In experiments on mice, it was observed that when deuterium levels increased by 3%, the birth weight of offspring decreased by 20%. Their size was three times smaller than the control group, and they could not reproduce beyond three generations. In another experiment, mice that consumed melted glacier water showed greater sexual activity and grew healthier than the control group. These experiments were replicated using other animals and plants in Soviet state laboratories. Considering that deuterium had been discovered just 30 years ago, this was a highly significant achievement. Thus, one of the secrets of longevity was finally revealed.
Coincidentally, around the same time, one of the biggest breakthroughs in biology was made by Paul D. Boyer, a molecular biologist at UCLA. He discovered that deuterium places a strain on the tiny protein nanomotors in the mitochondria, which produce ATP at the end of the electron transport chain (ETC). This protein assembly rotates at 9,000 RPM and possesses the structure and function of a mechanical motor, with a rotor, stator, and magnetic field. Boyer named it “ATP Synthase.”
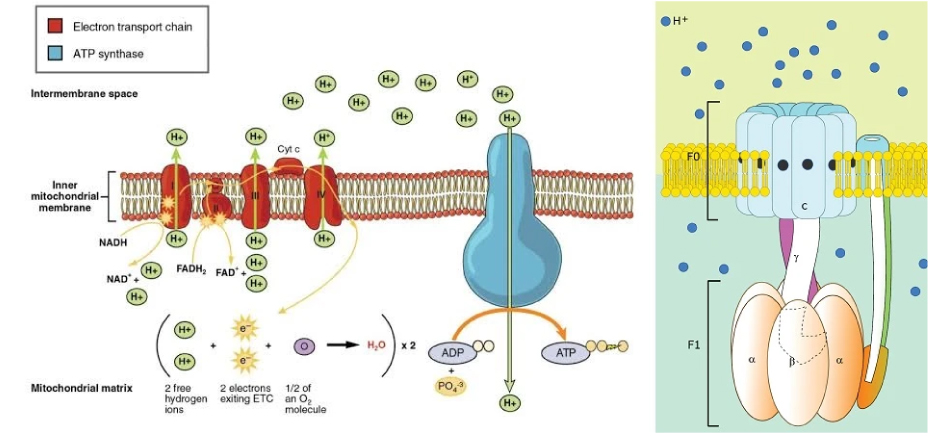
Enzyme Mechanism of the ATP Molecule
It took another 40 years for the effects of deuterium on ATP Synthase to be more clearly understood. Until the early 1960s, deuterium was not considered “completely different” in biochemical or biophysical terms than normal hydrogen; it was simply seen as having an additional neutron that made it twice in mass, but nothing else. However, this actually caught attention because no other element has such a large mass difference between its isotopes. The specific function of deuterium at the cellular level was yet to be revealed.
While the Russians continued their quiet research, Americans focused on studying the biological traces of deuterium. In 1963, John F. Thomson from the medical research division of the Argonne National Laboratory in Illinois wrote a 152-page paper titled “Biological Effects of Deuterium.” His colleagues, Joseph J. Katz and Henry L. Crespi, emphasized the biological significance of deuterium for the first time in their 1966 publication Deuterated Organisms Cultivation and Uses, which mentioned how deuterium affects the structure of proteins and DNA replication.
They studied the effects of water with increased or decreased deuterium levels on living organisms, and experiments on mice yielded the following results:
Experiment #1: Mice that drank 30% increased deuterium water showed a reduction in body weight and suffered fatal consequences within a few days.
Experiment #2: Mice that drank 30% reduced deuterium water (105 ppm) exhibited a 30% reduction in deuterium levels in their body fluids, leading to a significant increase in lifespan.
These studies suggest that deuterium can have a significant impact on the physiological processes of living organisms, particularly showing that water with lower deuterium levels may contribute to extending the lifespan of living beings.
Ten years later, in 1974, British scientist TR Griffiths presented a theory at the Second International Conference on Stable Isotopes at the Argonne National Laboratory, suggesting that deuterium could be a major cause of aging. He explained the role of deuterium in the progression of aging and other biochemical mechanisms, stating that “deuterium negatively affects the shape of enzyme molecules involved in DNA replication.” Griffiths observed that deuterium, being more electronegative, twice as heavy, and with different atomic bonding properties than hydrogen, interferes with DNA replication. He announced that an increase in deuterium could cause DNA repair enzymes to malfunction, potentially disrupting DNA replication and repair processes.
The following year, in 1975, JD Gleason and I. Friedman published the first U.S. study on promoting grain growth using deuterium-depleted water (DDW), based on plant growth research conducted in Russia.
The water in the Pakistani village of Hunza has a deuterium concentration reduced by approximately 16%, which may initially seem insignificant. However, according to Griffiths’ theory, the biological adverse effects of deuterium are proportional to the square of its concentration. This is an important finding, suggesting that even a slight reduction in deuterium can yield significant biological benefits. Following this theory, extensive research was conducted in Romania and Hungary throughout the 1990s.
These studies delved deeply into the effects of deuterium on aging and biological processes, suggesting that controlling deuterium levels may have positive effects on longevity and health.

Karakoram Mountains, Pakistan
W. Bild and his colleagues from the University of Medicine and Pharmacy in Romania published research showing that mice exposed to sub-lethal doses of 8.5 grays of radiation had higher survival rates when given deuterium-depleted water. Mice given water with a 30 ppm reduction in deuterium showed a 61% survival rate, while the control group, which drank regular tap water (150 ppm), had a 25% survival rate.
Additionally, after infecting them with pneumonia, the experimental group maintained normal levels of white blood cells, red blood cells, and platelets, and demonstrated stronger immune defenses than the control group.
Scientists concluded that the mice that drank deuterium-depleted water made fewer errors during cell division and more effectively repaired the DNA damaged by radiation, contributing to their survival.
At the time, the biological effects of deuterium-depleted water were scarcely known. While these findings seem miraculous already, they continued to demonstrate the significant biological impact of deuterium reduction. They conducted additional animal experiments to evaluate the effects of deuterium depletion on patients undergoing chemotherapy for cancer.
Later, in the early 1990s, Hungarian Nobel laureate Albert Szent-Györgyi and molecular biologist Gabor Somlyai conducted the most extensive clinical trials on deuterium depletion. Somlyai published the results of a double-blind clinical trial in his book The Biological Effects of Deuterium Depletion (1998) and in Defeating Cancer (2001), showing that deuterium-depleted water significantly improved cancer patient survival rates without side effects. He proved that deuterium-depleted water could be an excellent complementary treatment to radiation and chemotherapy.
From October 1992 to spring 1999, Dr. Somlyai and his team recorded more than 12,000 pages of documents, filed 1,200 patents, and administered over 350 tons of deuterium-depleted water to patients. By 2019, with 2,222 case studies on deuterium depletion, Somlyai’s groundbreaking research had positioned Hungary as a leading country in deuterium depletion research.
After the collapse of the Soviet Union, Berdyshev, the head of the Department of Genetics at Taras Shevchenko National University in Kyiv, launched a new department of Juventology. He developed equipment that could isolate deuterium by repeated freezing and thawing cycles. This tool could reduce deuterium concentration to 30-40% (90-105 ppm) and recreate melted glacial water. He also informed people without access to deuterium-depleted water that they could achieve similar results using a home freezer.
This method gained popularity in Russia and other countries starting in the 1990s, but it was deemed to be of limited effectiveness because it couldn’t reduce deuterium levels enough to make a significant difference.
By the early 21st century, researchers were well aware that deuterium-depleted water played an important role in protecting against DNA damage. However, the exact reasons were not yet fully understood.
In 2006, Russian chemist Igor A. Pomytkin and his colleague OE Kolesova published research on the relationship between mitochondrial deuterium concentration and the rate of hydrogen peroxide (H2O2) production. Their study showed that deuterium damages cells, while light water (deuterium-depleted water) supports health through mechanisms occurring in the mitochondria.
Pomytkin’s research indicated that deuterium suppresses the mitochondria’s ability to produce hydrogen peroxide (H2O2), which acts as a messenger molecule that regulates oxidative stress signals. His research reached the same conclusion as a study published the following year regarding the effects of deuterium on ATP synthesis.
These studies, all significant discoveries related to the biological effects of deuterium depletion, reflect humanity’s relentless pursuit to unlock the mysteries of the human body.

The year 2007 marked one of the most monumental discoveries in the short history of deuterium science. Abdullah Olgun, a doctor, biochemist, and pharmacologist at Gülhane Military Medical Academy’s Faculty of Health Sciences in Ankara, Turkey, presented the biological effects of deuterium and aging, using ATP Synthase as an example.
He dedicated himself to studying the mechanisms that cause aging and immersed himself in two years of research. To demonstrate how deuterium causes damage, he even pursued another degree in medical mathematics. Olgun confirmed that ATP Synthase occurs at the final stage of the electron transport chain within the nanomotor.
According to Olgun’s research, deuterium causes proton-neutron pairs without open receptors to collide with the rapidly rotating nanomotor approximately every 15 seconds, eventually leading to a breakdown. He detailed this in his 2007 paper Deuteronation and Aging and presented it the same year in the Annals of the New York Academy of Sciences, identifying it as one of the primary causes of aging. This discovery finally unlocked the mystery of how deuterium causes damage to life. Olgun’s findings are considered one of the greatest discoveries in 21st-century biology and a rediscovery of an overlooked biological mechanism.
The person who applied this discovery practically was Anton Chernopiatko, a Russian businessman, scientist, and researcher of deuterium-depleted water. In 2015, he co-authored a study with Russia’s Igor A. Pomytkin and Oxford scientists showing that increased deuterium content correlates with heightened susceptibility to depression, exploring the “potential role of serotonin-related mechanisms.” Chernopiatko, who had recognized the importance of deuterium depletion since his student days, devoted his life to research, and his work was beginning to bear fruit.
Today, Chernopiatko is focused on building a commercial-scale factory to produce light water, applying the extensive knowledge of deuterium’s biological role beyond laboratories and research settings. Although Berdyshev developed an industrial process in the 1990s to reduce deuterium by 30-40% using refrigeration methods, the first vacuum purification system for producing light water was developed by Dr. Igor A. Pomytkin. These advancements demonstrate the commercial significance of deuterium-depleted water, opening a new chapter in deuterium research.
Selivanenko from the Moscow Institute of Fine Chemical Technology developed a much more efficient process over decades of research, capable of removing more than 97% of deuterium. In 2008, Anton Chernopiatko acquired the intellectual property from Selivanenko and worked with engineer Alexander Emalianov for several years to improve it. As a result, in 2012, they commercialized the technology developed at the Moscow Institute, leading to the construction of a deuterium-depleted water production plant.
While the process and production systems for removing deuterium are already known, improvements for more economical and efficient methods are still needed. Additionally, public awareness of deuterium-depleted water remains in its very early stages.
The four international conferences on deuterium depletion held in Budapest provided a crucial platform for scientists to present their research. One of the leading American scientists in this field is Dr. Laszlo Boros, a professor and medical doctor at the University of California. He is likely one of the most knowledgeable biochemists in the world in this field. Boros co-founded the Center for Deuterium Depletion in Los Angeles, making deuterium testing and treatment protocols available to the public for the first time.
Many scientists in this field argue that it is desirable to maintain the body’s ideal deuterium level below 120 ppm. To achieve this, drinking 1.5 liters of deuterium-depleted water (with 80-100 ppm) daily for 45-60 days while minimizing the intake of other beverages can prevent diseases and promote longevity. For those already suffering from illnesses, this can help cure diseases. After 60 days, maintaining this level by consuming deuterium-depleted water in the 100-120 ppm range is recommended.
In Korea, Hue Light, in cooperation with the Moscow Institute of Fine Chemical Technology, has become the first to supply deuterium-depleted water to cancer patients.
I wrote this article driven by my constant curiosity and thirst for medical knowledge. It is a great honor to have studied and summarized the literature of all doctors and scientists mentioned in this article, and be the first to introduce this topic to South Korea.





