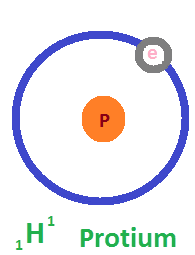
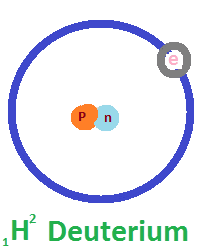
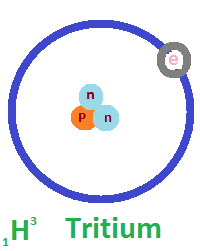
In a 75 kg human body, which contains about 50 kg of water, approximately 10 grams of deuterium are naturally present. While deuterium occurs in small, safe amounts, physiological studies have shown that high concentrations can be harmful—infertility may occur when 25% of the body’s water contains deuterium, and survival drops to about a week at 50%. Aquatic organisms like fish and tadpoles cannot survive in environments with 90% deuterium water. Despite these biological effects at high levels, deuterium is highly valuable in scientific and industrial fields. It is used to slow down neutrons in nuclear reactors, supports detailed imaging in Nuclear Magnetic Resonance (NMR) spectroscopy, and plays a key role in producing blue light sources in OLED technology. Today, deuterium-depleted water (DDW), with concentrations as low as 5 ppm, is also gaining attention in health, clinical, and research applications due to its unique properties.
Hue Light’s Deuterium Depleted Water (DDW) offers one of the lowest deuterium concentrations available, providing a pure hydration solution aimed at enhancing cellular function and overall wellness.
- Ultra-Low Deuterium Levels: Available in concentrations as low as 5 ppm, significantly lower than the typical 145–155 ppm found in regular water.
- Supports Cellular Energy: Reduced deuterium levels may help optimize mitochondrial function, potentially enhancing energy production at the cellular level.
- Natural Composition: Maintains essential minerals and organic matter, ensuring the water’s natural integrity while reducing deuterium content.
- Multiple Packaging Options: Available in various sizes, including 500ml (5 ppm), 1L (78 ppm), and 2L (10 ppm), to suit different consumption needs.